Abstract
Background:
Intravenous (IV) iron infusions are associated with a variety of adverse drug reactions (ADRs) including anaphylactic reactions. The Commonly used IV iron formulations are Iron sucrose (Venofer), Ferric carboxymaltose (Injectafer), Sodium ferric gluconate complex (Ferrlecit), and others. Iron sucrose has been approved by the FDA (Food and Drug Administration) for patients with iron deficiency who also have kidney disease. It is also often used off-label for iron deficiency anemia on its own. In our facility, multiple ADRs were noted with Venofer compared to other formulations. Hence, we aimed to study: 1. If altering the dose of Venofer will result in a lesser incidence of allergic reactions. 2. To determine if one preparation of IV iron has a higher incidence of adverse events than another. 3. To evaluate the efficacy after 6 to 8 weeks of completion of iron infusions, by comparing the response to various dosages and formulations.
Methods:
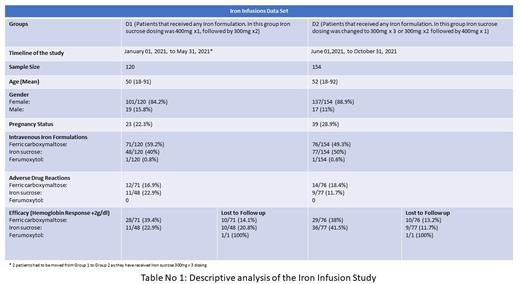
This is a retrospective chart review of 274 patients ages 18-92 who received at least one dose of Intravenous Iron at Einstein Medical Center Montgomery Outpatient Infusion Center from 01/01/21 to 10/31/21. Patients were divided into two groups based on the Venofer dosing schedule. Group D1 included patients that received any IV iron formulation from January to May 2021. In this group, Venofer dosing was 400mg x 1 followed by 300mg x2. Multiple ADRs were noticed in group D1 hence Group D2 was created. This group included patients that received any IV iron formulation from June to October 2021, where Venofer dosing was changed to 300mg x 3 doses or 300mg x 2 followed by 400mg. We collected data on age, gender, race, preparation, strength, pregnancy status, prior history of allergies and transfusion reactions, adverse drug reactions, the timing of reaction, total doses received, pre/post-medications provided to prevent or treat a reaction, outcomes of reaction, hemoglobin, iron studies before and after the infusions. Patient characteristics in both D1 and D2 groups were well balanced. Data were analyzed and summarized in descriptive measures. Efficacy of intravenous iron treatment was measured as a rise in Hemoglobin by 2gm/dl.
Results:
Out of 120 patients in group D1, 71 patients (59.2%) received Injectafer, out of these, 12 patients (16.9%) had ADRs, the most common reaction was hypotension, and 1 patient had an anaphylactic reaction requiring Emergency Room (ER) visit. Responders in this group were 28/71(39.4%). 10/71 (14.1%) were lost to follow-up. 48 patients (40%) received Venofer, out of which 11 patients (22.9%) had ADRs, the most common being pain in the back, and 2 patients required ER visits. Responders to the infusion in this group were 11/48 (22.9%) with 10/48 (20%) lost to follow-up. 1 patient (0.8%) received ferumoxytol, with no ADRs to report.
In the D2 group, which involved a total of 154 patients, 76 patients (49.3%) received Injectafer, out of which 14 patients (18.4%) had ADRs, again the most common being hypotension, and 2 patients required an ER visit. Responders in this group were 29/76 (38.1%) and 10/76 (13.2%) were lost to follow-up. 77 patients (50%) received Venofer, out of which 9 patients (11.7%) had ADRs, the most common being hypotension and this group has not had any ER visits. Responders to the infusion in this group were 36/77(46.8%) and 9/77 (11.7%) were lost to follow-up. 1 patient (0.6%) received Ferumoxytol with no ADRs. [See Table 1].
Conclusions:
Injectafer was overall the most used iron formulation in our institution. It was associated with more ADRs when compared to Venofer, including anaphylactic reactions. The most common ADR was hypotension with Injectafer, and back pain with Venofer. We found that Venofer dosing with 400mg x 1 followed by 300mg x2 was associated with the highest incidence of ADRs. Both strategies of Venofer dose modification with 300mg x 3 and schedule modification, 300x 2 followed by 400mg had greater efficacy and lesser frequency of ADRs as compared to Venofer 400mg followed by 300mg x2. Other studies have shown the benefit of Venofer in non-renal disease patients with iron deficiency. Although our study was not planned to demonstrate this outcome, our data may suggest that Venofer is beneficial for this group of patients. This should be looked into with further studies.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Iron sucrose is FDA (Food and Drug Administration) approved for anemia and chronic kidney disease(CKD) with dosage of 100 mg during dialysis session for hemodialysis dependent patients, 300 mg x 2 followed by 400 mg x 1 for peritoneal dialysis patients. In Non dialysis dependent CKD patients the approved dosing is 200 mg x 5. Depending on the insurance and the available formulations, iron sucrose has been historically also being used off label for chemotherapy associated anemia, iron deficiency anemia without CKD. The dosage formulations that are being used in various institutions are quite varied. On literature review, we came across the study from 2008 where the dosing was calculated using any equation that included the desired hemoglobin value, observed hemoglobin, ideal body weight and sex. Calculated dose was divided into portions found her to the nearest 250 mg, administered over 4 hours every other day. During this regimen, if the total dosage of iron required was 1250 mg, they were given 500 mg on day 1, 500 mg on day 3 and 250 mg on day 5. And they have noted that this was well tolerated. This was published in Journal of pharmacy practice in 2021. In other institutions, dosing with 500 mg iron sucrose x2, 200 mg iron sucrose x5, 300 mg iron sucrose x3 have also been used. Most of these dosages are pretty well-tolerated.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal